Alzheimer's Antibody Drugs: What You Really Need to Know
An evidence-based review for individuals experiencing memory changes and those caring for them
Quick Navigation
What Are Monoclonal Antibody Drugs?
How Do They Compare to Donepezil?
Numbers That Matter: NNT & NNH
Monoclonal antibody drugs such as lecanemab (Leqembi) and donanemab (Kisunla) are intravenous treatments designed to target amyloid-beta, a protein that accumulates in the brains of people with Alzheimer’s disease. These drugs aim to reduce amyloid buildup, which researchers believe plays a role in disease progression.
Unlike older Alzheimer’s medications that temporarily support brain signaling, monoclonal antibodies attempt to change one biological feature of the disease. However, reducing amyloid does not necessarily translate into meaningful improvements in memory or daily functioning.
Clinical trials show that people taking lecanemab or donanemab continue to experience cognitive decline over time. While these drugs slowed decline slightly compared with placebo, the differences were very small.
Key findings from trials:
- Decline occurred in all groups, including those on treatment
- After ~18 months, the difference between drug and placebo was minimal
- Changes were often below levels considered noticeable in daily life
What this means in real life: Most patients and caregivers would not perceive a meaningful difference in memory, independence, or quality of life.
Donepezil (Aricept) has been prescribed for over 20 years and is taken as a daily pill.
Feature
| Donepezil (Aricept)
| Antibody Drugs
|
| How it’s taken | Oral pill | IV infusion |
| Effect | Modest, temporary | Very small slowing of decline |
| Safety | Well-studied | Higher risk profile |
| Monitoring | Minimal | Frequent MRIs |
When cognitive scores are plotted on the same scale, donepezil shows equal or greater short-term benefit compared with monoclonal antibodies.
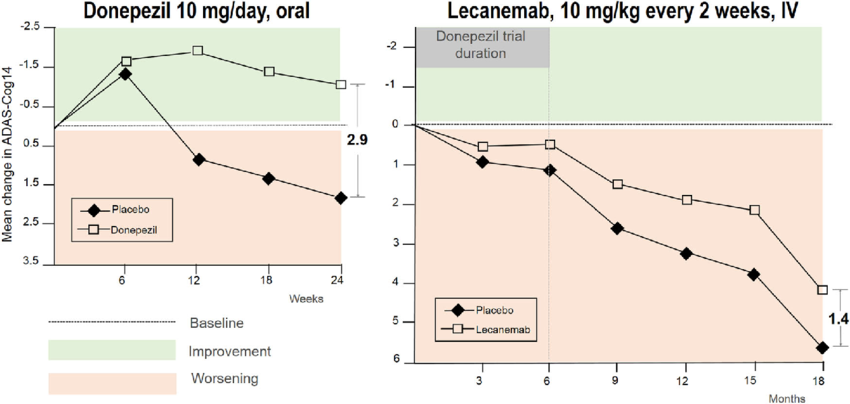
The most significant concern with monoclonal antibodies is safety.
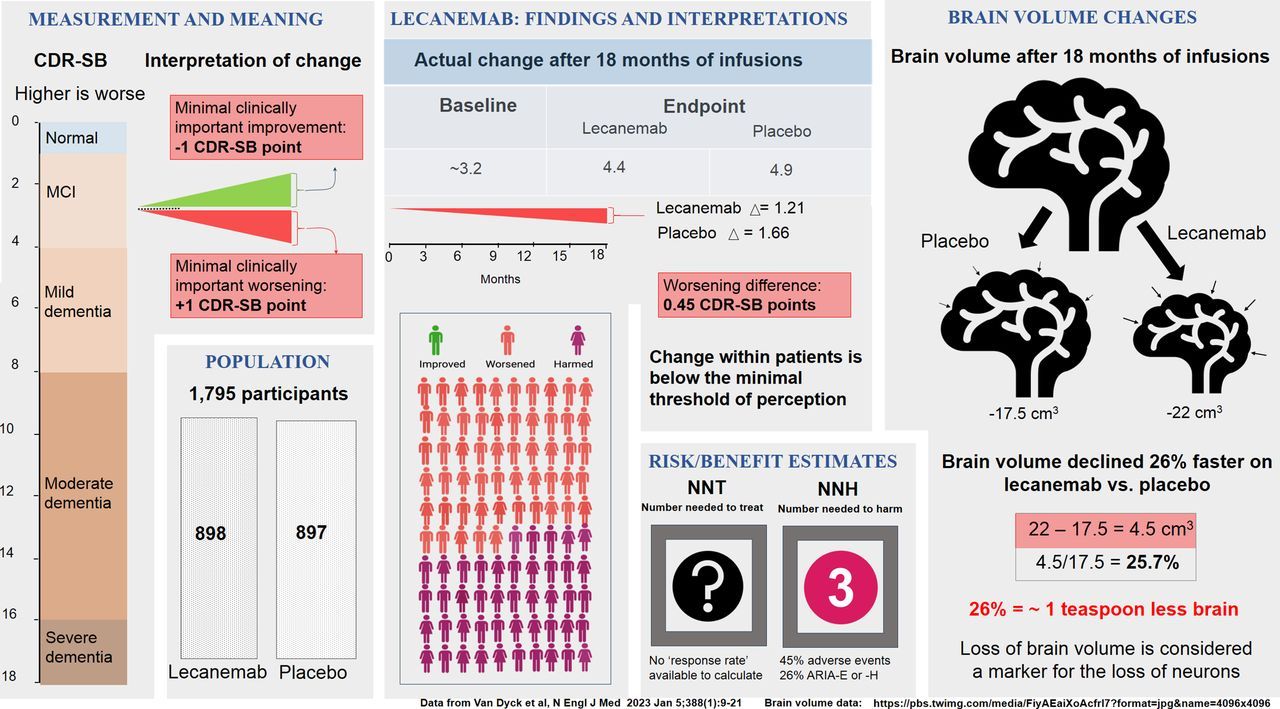
Lecanemab
- ~46% experienced drug-related side effects
- ~25% developed brain swelling or bleeding (ARIA)
- Severe cases and deaths have been reported
Donanemab
- ~88% experienced side effects
- 35% developed ARIA
Brain swelling may accelerate brain shrinkage, which is already a core problem in Alzheimer’s disease.
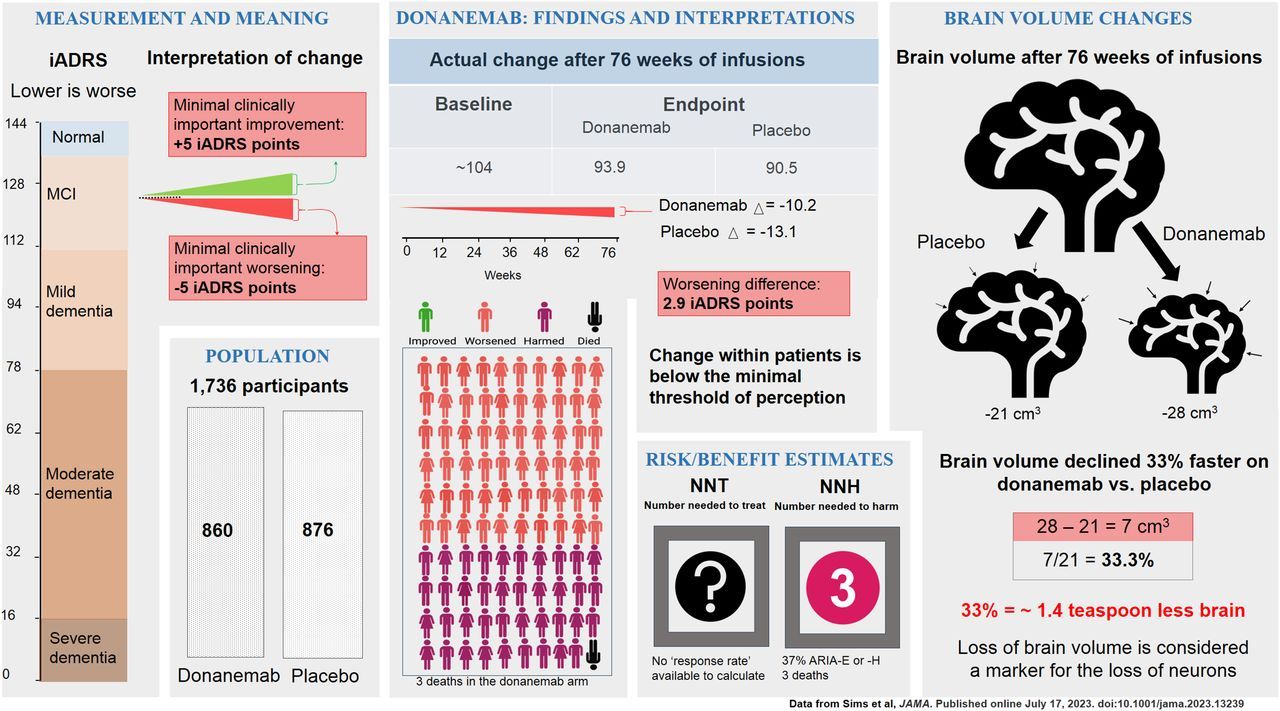
Number Needed to Harm (NNH): ~3
For every 3 people treated, 1 experiences a significant harmful side effect.
Number Needed to Treat (NNT): Unknown
Trials have not shown a clear, meaningful benefit at the individual patient level.
Monoclonal antibody treatments also carry a significant financial burden, both for individuals and for the broader health care system.
Drug cost alone: Lecanemab and donanemab are priced at approximately $25,000–$32,000 per person per year.
Additional medical costs: Because these drugs require regular IV infusions, frequent MRI brain scans, specialist visits, and monitoring for side effects, the true annual cost per patient is substantially higher.
Out-of-pocket impact: Even with insurance or Medicare coverage, patients and families may face thousands of dollars per year in copays, deductibles, travel time, and caregiver burden.
System-wide impact: If widely adopted, these therapies would place billions of dollars of strain on the U.S. health care system each year, raising concerns about sustainability given their very small clinical benefit.
These costs must be weighed alongside the modest slowing of decline and the real risks of harm.
- These drugs do not improve memory or thinking
- They slightly slow decline, but the effect is very small
- Risks, costs, and treatment burden are substantial
For many patients, older therapies like donepezil may be safer and at least as effective in the short term, even though benefits remain modest.
Need help deciding what this evidence means for you or your loved one?
A pharmacist can help you weigh benefits, risks, costs, and practical considerations — without pressure.
👉 Talk With a Pharmacist About Your Options
Only people with very early Alzheimer’s disease and confirmed amyloid buildup may qualify.
Van Dyck CH, et al. Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine. 2023;388(1):9–21.doi:10.1056/NEJMoa2212948
Sims JR, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. New England Journal of Medicine. 2023;388(1):1–13.doi:10.1056/NEJMoa2300709
Cummings J, et al. Aducanumab: Appropriate Use Recommendations. Journal of Prevention of Alzheimer’s Disease. 2021;8(4):398–410.doi:10.14283/jpad.2021.41
U.S. Centers for Medicare & Medicaid Services (CMS). Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease — Coverage Policy. Updated 2023.
Lin GA, et al. Cost-effectiveness of Lecanemab for Individuals With Early Alzheimer Disease. JAMA Neurology. 2023;80(3):1–9. doi:10.1001/jamaneurol.2022.4798
Institute for Clinical and Economic Review (ICER). Lecanemab for Early Alzheimer’s Disease: Effectiveness and Value. Final Evidence Report. 2023.
Knopman DS, et al. Amyloid-Related Imaging Abnormalities With Anti-Amyloid Monoclonal Antibodies. Alzheimer’s & Dementia. 2021;17(6):1–12. doi:10.1002/alz.12225
U.S. Food and Drug Administration (FDA). Leqembi (lecanemab-irmb) Prescribing Information. Revised 2023.
Rabins PV, et al. Practical Considerations in the Use of Disease-Modifying Therapies for Alzheimer’s Disease. Neurology. 2023;100(9):1–10.doi:10.1212/WNL.0000000000201571
Cubanski J, Neuman T. Medicare Spending and Financing Implications of New Alzheimer’s Drugs. Kaiser Family Foundation. 2023.



